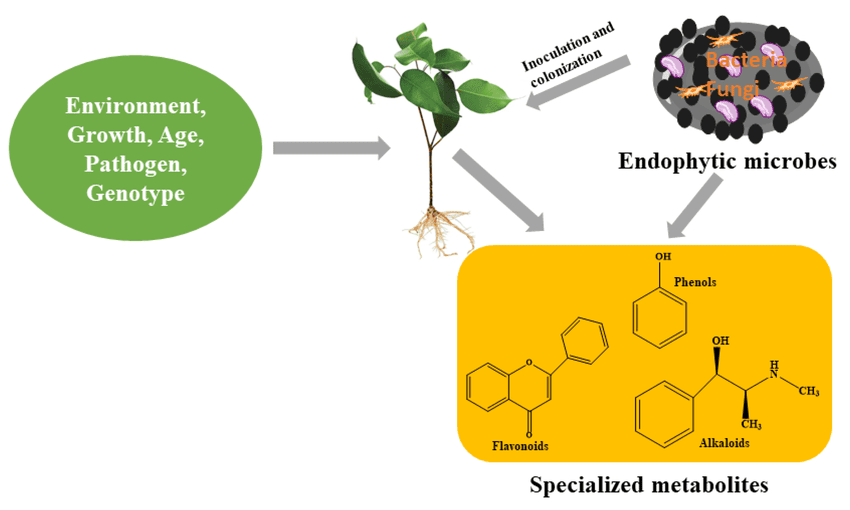
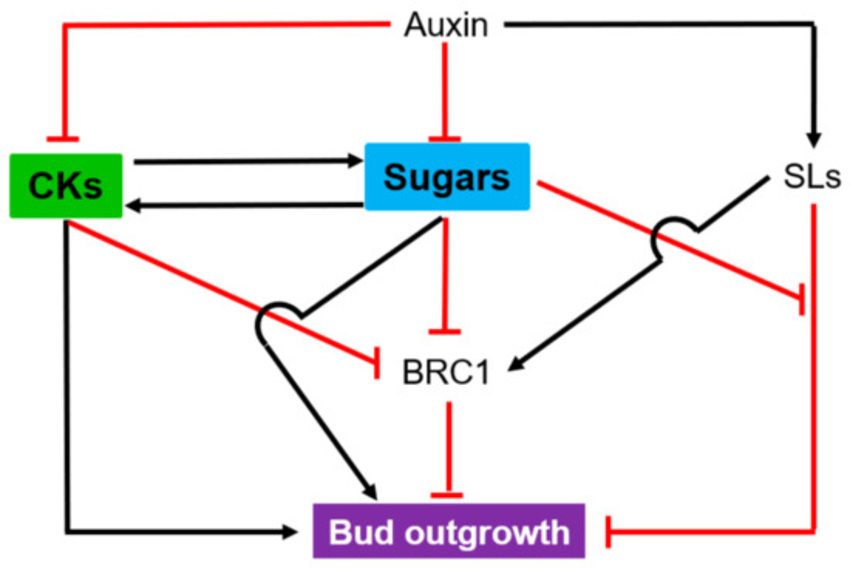
Plant Molecular Biology
52
Aging could be slowed down by controlling the stress response in cells.
- Rating
- elderly
- diseases
- aged
- biological
- dementia
- diabetes
Researchers at Nanyang Technological University in Singapore (NTU Singapore) discovered that turning on a stress response in cells at a post-reproductive age may be the secret to delaying aging and extending life.
The NTU Singapore team conducted lab tests on a species of roundworm that is comparable to people and discovered that feeding old worms a high-glucose diet prolonged their lifetime in comparison to worms fed a normal diet by turning on this stress response in these worms.
According to the NTU team's findings, which were published in Nature Communications, this is the first time a connection between this stress response and aging has been discovered.
The scientists said their findings open the door to the creation of medicines that could delay the onset or even combat age-related illnesses like cancer, dementia, and stroke, however further research is required to fully grasp this connection.
Associate Professor Guillaume Thibault, a cell biologist and the study's principal investigator from the NTU School of Biological Sciences, claims that "Aging is a significant risk factor for a number of human pathologies, including cancer and neurodegenerative diseases as well as metabolic diseases like diabetes. Finding the molecular mechanisms underlying the aging process could advance the development of new therapeutic approaches to treat age-related diseases from the perspective of public health."
"While our research indicated that a high-glucose diet might help elderly worms live longer and age more slowly, we do not advocate that the elderly switch to a high-sugar diet at this time. What this study does demonstrate is that a drug-induced stress response may be essential for slowing cellular aging and may contribute to lifespan by inducing specific stress responses in cells."
The NTU researchers not only demonstrated the effects of modifying this stress response in older worms, but they also demonstrated how turning off the same response in young worms fed a high-glucose diet enabled them to survive longer than worms on a regular diet.
Professor Rong Li, Director of the Mechanobiology Institute at the National University of Singapore, makes the following observations in his capacity as an impartial expert: "If untreated, metabolic illnesses in the elderly have substantial side effects. The significance of this research lies in the discovery of a biological process, known as the unfolded protein response, that influences lifespan in animals fed a high-glucose diet."
"They discovered that severely extending the lifespan of these animals by blocking this system. Therefore, they suggest that blocking this route might increase lifespan in those with metabolic disorders."
The NTU2025 five-year strategic plan, which focuses on health and society as one area with the potential for major intellectual and societal influence, is in line with this study's research pillar.
How the stress response is triggered in cells
When stresses (such as an abundance of glucose) result in a build-up of harmful "unfolded" proteins in the cell, the cell responds by producing a stress response. The unfolded protein response, or stress response, attempts to eliminate these troublesome proteins and reestablish cellular equilibrium.
Due to a natural loss in the capacity of the cell's machinery to create healthy proteins, aging may also result in a buildup of unfolded proteins, which would cause the similar stress response.
The "stress sensors" in the cell's molecular machinery deal with this build-up by triggering a number of molecular reactions to protect the cell from the stress. The prolonged unfolded protein response instead causes cell death if the overabundance of unfolded proteins is not reduced.
Aged worms' sensitivity to unfolded proteins resulted in better aging
The scientists used glucose to induce the unfolded protein response in adult roundworms (Caenorhabditis elegans) in order to study how this response impacts animal longevity. Even while C. elegans has a far simpler physical structure than a person, it depends on many of the same genes as humans to regulate cell division and instruct defective cells to die.
When the worms were young, that is, at the beginning of maturity (Day 1), and at a post-reproductive age, that is, when they were old and no longer fertile (Day 5), the scientists fed some of the worms a high-glucose diet. All the while, a control group of worms had a regular diet.
The researchers discovered that the longevity of old worms fed a high-glucose diet was 24 days, about twice as long as that of young worms fed the same diet (13 days). Worms survived for 20 days on a typical diet.
Compared to worms fed a normal diet, the older worms on a high-glucose diet were more nimble and had more energy storage cells, suggesting healthier aging. They also lived longer.
Young worms' protracted stress response resulted in cell death.
The NTU researchers observed the activity of the three stress sensors, each of which controls a distinct cellular pathway in the unfolded protein response, a day after giving the worms a high-glucose diet.
One of the stress sensors, IRE1, was discovered to be substantially more active in young worms than in older worms.
Young worms on a high-glucose diet starting on Day 1 survived for 25 days—twice as long as when the IRE1 gene was intact. This was discovered when researchers deleted the gene coding for IRE1 from worms to "turn off" the cellular pathway the stress sensor begins.
This shows that the shortened lifetime of newborn worms fed a high-glucose diet from Day 1 was caused by the elevated activation of the stress sensor IRE1, which the researchers refer to as a prolonged unfolded protein response.
They "believe that the high-glucose diet fed to the aged worms stimulated their otherwise sluggish unfolded protein response and switched on certain cellular pathways, tackling not only the stress caused by excess glucose but also other aging-related stress, restoring cellular stability," according to Assoc Prof Thibault.
"Young worms on a high-glucose diet, however, caused unresolved stress in the cells as a result of an overactive IRE1. The cells instead started cell death as a result of the extended stimulation."
According to the research, a medication that increases the activity of the other two stress sensors while decreasing the activity of IRE1 may one day be created to slow down cellular aging and so lengthen lifespans.
To further understand the intricate mechanism underlying the lifetime extension brought on by a high-glucose diet, as well as how this mechanism interacts with other processes in cells, more research and findings must be made in the context of roundworms.
Leave a Reply
Your email address will not be published. Required fields are marked *